We can't find the internet
Attempting to reconnect
Something went wrong!
Attempting to reconnect
Product Information
Indication
Hyperacidity, burning of chest, bleaching acidic, Dyspepsia, such digestive disorder and pre gastric ulcer stages.
Composition
Capsicum annum 30C, Cinnamomum 30C, Carbo Vegetabilis 30C, Acidum muriaticum 30C
Dosage
One teaspoonful once a day one hour before lunch.
Available Packing
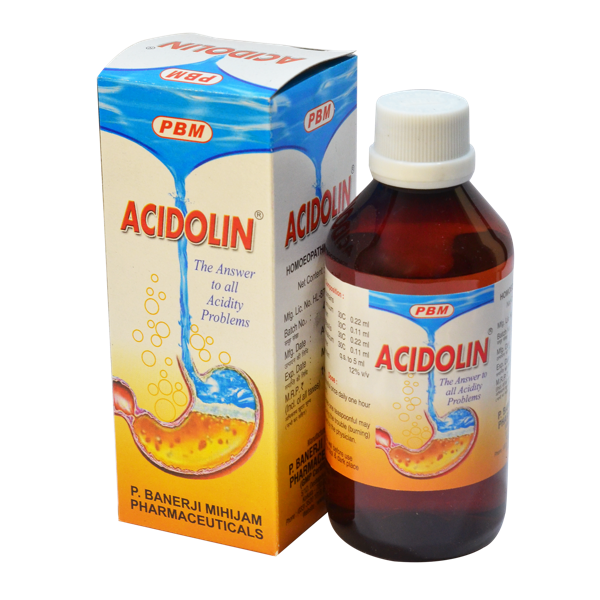
Detailed Information
Acidolin — Trade/Clinic Specification (India)
AYUSH-GMP • 100 ml / 200 ml • Professional Use • Flammable (ethanol base)
For registered practitioners, clinics, and pharmacies. This page provides specification, compliance, logistics, and documentation details. No therapeutic claims are made beyond the approved label.
Product Overview
Acidolin is a proprietary homeopathic formulation prepared under AYUSH-GMP. This page summarizes label indications, composition, packaging, quality documentation, and logistics for professional supply.
Label Indications (verbatim)
- Hyperacidity
- Burning sensation in chest and epigastric region
- Acidic belching
- Dyspepsia (indigestion)
- Pre-gastric-ulcer stage
Use consistent with the product label and applicable regulations.
Dosage (label)
- Adults: One teaspoonful (≈ 5 ml) once daily, one hour before lunch, or as directed by a qualified practitioner.
Composition & Potency
Actives (per label):
- Capsicum annuum 30C
- Cinnamomum 30C
- Carbo vegetabilis 30C
- Acidum muriaticum 30C
Vehicle: Pharmaceutical-grade ethanol + purified water
Manufacturing standard: Prepared per HPI in an AYUSH-GMP facility.
Packs & Trade Data
| Pack size | SKU | GTIN-13 / EAN |
|---|---|---|
| 100 ml | ACID-100 |
________________ |
| 200 ml | ACID-200 |
________________ |
Label elements present: Product name, composition, potency, batch no., Mfg/Exp dates, storage, cautionary statements, manufacturer details.
Case Pack & Carton Logistics
| Pack size | Units / master carton | Carton dimensions (cm) | Gross wt. (kg) |
|---|---|---|---|
| 100 ml |
24 |
__ × __ × __ |
__.__ |
| 200 ml |
12 |
__ × __ × __ |
__.__ |
- Palletisation: Specs available on request
-
HSN:
3004.90(confirm at dispatch) -
Ethanol (v/v):
__%(per label)
Storage & Handling
- Store below 25 °C, protected from light.
- Keep container tightly closed; keep away from strong odours and heat sources.
- Flammable (ethanol base).
- Keep out of reach of children.
Flammable liquid: Keep away from heat/sparks/open flames. No smoking. Store in a well-ventilated area.
Regulatory & Compliance
- AYUSH-GMP: Yes
- Manufacturing documentation: Master formula, batch manufacturing records, change control retained per QMS
- Label compliance: As per Drugs and Cosmetics rules (India); includes mandatory cautionary statements and storage
- Intended audience: Registered practitioners, licensed clinics/hospitals, authorized pharmacies/dispensaries
Quality Control & Documentation
Batch documentation available to professional customers (on request / behind login):
- COA (Certificate of Analysis) per batch
- MSDS/SDS
- Stability summary (where applicable)
- Traceability documentation
Routine QC (summary):
- Identity checks (source verification)
- In-process controls per HPI/QMS
- Finished product checks: organoleptic, ethanol % (v/v), label accuracy, traceability
Access documentation via the professional portal or request from QA with batch number.
Supply & Dispatch (Logistics)
- Cut-off for same-day dispatch (stocked lines): 14:00 IST
- Typical dispatch window: 1–5 business days India-wide (route dependent)
- Couriers (flammable compliant): XpressBees, BlueDart (routing may vary)
- Transport packaging: Shock-resistant outer; flammable handling labels as applicable
For shipment coordination and documentation support, contact the professional/QA desk with your batch number and destination details.
Marketing & Professional Materials
Available to professional customers (on request / behind login):
- High-resolution pack images (100 ml / 200 ml)
- Product specification sheet (PDF)
- Storage & handling poster (A4)
- Safety data sheet
Frequently Asked (Trade)
- How do I obtain a COA/MSDS? Provide the batch number to the QA desk or use the professional portal.
- What shelf-life do shipments carry? Shipments typically maintain ≥80% of labeled shelf-life remaining unless otherwise specified.
- Can I confirm GTIN/EAN for cataloguing? See the Packs & Trade Data table above; QA can re-confirm if needed.
Document Information
Last Updated: October 20, 2025 Document Version: 1.0 Review Date: May 20, 2026
Professional Contacts (documentation & QA):
- QA/Docs: docs@pbmp.in (include product + batch no.)
- General Information: info@pbmp.in
Disclaimer: This document is intended for healthcare professionals and authorized trade customers. It does not constitute medical advice. All information aligns with product labeling and applicable regulations. Use per professional judgment and local law.