We can't find the internet
Attempting to reconnect
Something went wrong!
Attempting to reconnect
Product Information
Indication
Sore throat, hoarseness of voice due to common cold, congestion of lung, heaviness of frontal head, difficulty in expectoration.
Composition
Hepar sulphur 30C, Bryonia alba 30C, Kalium muriaticum 3X, Honey
Dosage
1 teaspoonful twice daily morning and evening. After improvement once a day until recovery.
Available Packing
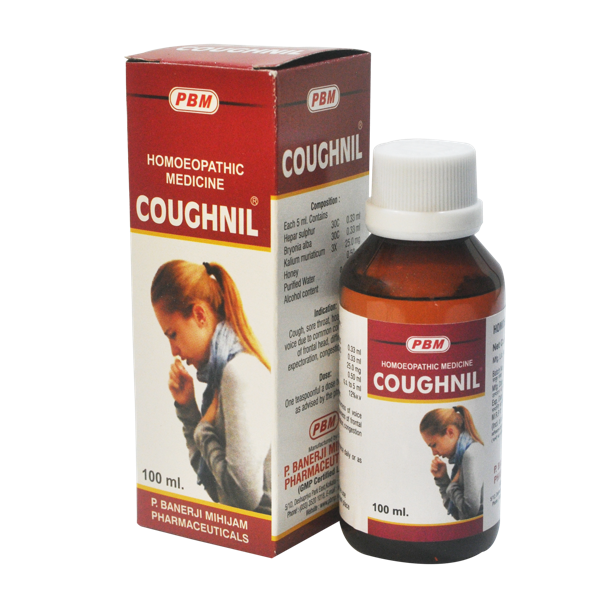
Detailed Information
Coughnil — Trade/Clinic Specification (India)
AYUSH-GMP • 100 ml • Professional Use • Flammable (ethanol base)
For registered practitioners, clinics, and pharmacies. This page provides specification, compliance, logistics, and documentation details. No therapeutic claims are made beyond the approved label.
Product Overview
Coughnil is a proprietary homeopathic formulation prepared under AYUSH-GMP. This page summarizes label indications, composition, packaging, quality documentation, and logistics for professional supply.
Label Indications (verbatim)
- Sore throat
- Hoarseness of voice due to common cold
- Congestion of lung
- Heaviness of frontal head
- Difficulty in expectoration
Use consistent with the product label and applicable regulations.
Dosage (label)
- Standard: One teaspoonful (≈ 5 ml) twice daily morning and evening
- After improvement: One teaspoonful once a day until recovery, or as advised by the physician
Composition & Potency
Actives (per 5 ml label):
- Hepar sulphur 30C — 0.33ml
- Bryonia alba 30C — 0.33ml
- Kalium muriaticum 3X — 25.0mg
- Honey — 0.50ml
Vehicle: Pharmaceutical-grade ethanol + purified water + honey
Alcohol content: 12% v/v
Manufacturing standard: Prepared per HPI in an AYUSH-GMP facility.
Packs & Trade Data
| Pack size | SKU | GTIN-13 / EAN |
|---|---|---|
| 100 ml | COUG-100 |
________________ |
Label elements present: Product name, composition, potency, batch no., Mfg/Exp dates, storage, cautionary statements, manufacturer details.
Case Pack & Carton Logistics
| Pack size | Units / master carton | Carton dimensions (cm) | Gross wt. (kg) |
|---|---|---|---|
| 100 ml |
24 |
__ × __ × __ |
__.__ |
- Palletisation: Specs available on request
-
HSN:
3004.90(confirm at dispatch) -
Ethanol (v/v):
12%(per label)
Storage & Handling
- Store below 25 °C, protected from light.
- Keep container tightly closed; keep away from strong odours and heat sources.
- Flammable (ethanol base).
- Keep out of reach of children.
Flammable liquid: Keep away from heat/sparks/open flames. No smoking. Store in a well-ventilated area.
Regulatory & Compliance
- AYUSH-GMP: Yes
- Manufacturing documentation: Master formula, batch manufacturing records, change control retained per QMS
- Label compliance: As per Drugs and Cosmetics rules (India); includes mandatory cautionary statements and storage
- Intended audience: Registered practitioners, licensed clinics/hospitals, authorized pharmacies/dispensaries
Quality Control & Documentation
Batch documentation available to professional customers (on request / behind login):
- COA (Certificate of Analysis) per batch
- MSDS/SDS
- Stability summary (where applicable)
- Traceability documentation
Routine QC (summary):
- Identity checks (source verification)
- In-process controls per HPI/QMS
- Finished product checks: organoleptic, ethanol % (v/v), honey content verification, label accuracy, traceability
Access documentation via the professional portal or request from QA with batch number.
Supply & Dispatch (Logistics)
- Cut-off for same-day dispatch (stocked lines): 14:00 IST
- Typical dispatch window: 1–5 business days India-wide (route dependent)
- Couriers (flammable compliant): XpressBees, BlueDart (routing may vary)
- Transport packaging: Shock-resistant outer; flammable handling labels as applicable
For shipment coordination and documentation support, contact the professional/QA desk with your batch number and destination details.
Marketing & Professional Materials
Available to professional customers (on request / behind login):
- High-resolution pack images (100 ml)
- Product specification sheet (PDF)
- Clinical reference guide for respiratory conditions
- Storage & handling poster (A4)
- Safety data sheet
Frequently Asked (Trade)
- How do I obtain a COA/MSDS? Provide the batch number to the QA desk or use the professional portal.
- What shelf-life do shipments carry? Shipments typically maintain ≥80% of labeled shelf-life remaining unless otherwise specified.
- Can I confirm GTIN/EAN for cataloguing? See the Packs & Trade Data table above; QA can re-confirm if needed.
- Does this contain honey? Yes, the formulation includes 0.50ml honey per 5ml dose as per label composition.
- Is this suitable for chronic respiratory conditions? Yes, dosing can be adjusted from twice daily to once daily after improvement for continued management.
Document Information
Last Updated: October 23, 2025
Document Version: 1.0
Review Date: May 23, 2026
Professional Contacts (documentation & QA):
- QA/Docs: docs@pbmp.in (include product + batch no.)
- General Information: info@pbmp.in
Disclaimer: This document is intended for healthcare professionals and authorized trade customers. It does not constitute medical advice. All information aligns with product labeling and applicable regulations. Use per professional judgment and local law.